Chemical equations are of great importance for daily life since we can observe them in different substances that react with each other. Whether they are simple or compound, it is when the use of chemical symbology is warranted for each of the intervening solutions. Identifying in this way the elements that participate. In addition to using notations in numbers, particular signals that have to do with the combination(s) in the chemical equation.
What is a Chemical Equation
Definition Show the reacting substances and the substances that are produced. It is the writing of symbols for a reaction. It can understand that it is the equalization of atoms, molecules and particles, by the beginning or end of a reaction resulting from a chemical transformation. Help to the visualization of both the reagents and the product. Use symbols to identify the elements or compounds that are within the chemical equation. The first chemical equation was recorded in the year 1615 by Jean Bergin.
They are the expression of the changes that happen in the course of the chemical reaction. It consists of two parts, the reactants and the resulting substances. It can be interpreted in two ways, qualitatively considering the nature of the reactants. The quantitative, it conceives to take into account the relationships between molecules. Particles and the atoms. They are the interpretation of a chemical reaction. They are also the schematic and abbreviated representation of a reaction.
On what basis is a chemical equation balanced
A chemical equation is balanced based on the law of conservation of mass. This states that in a chemical reaction, the total mass of the reactants must equal the total mass of the products. Therefore, the number of atoms of each element must be the same on both sides of the equation. The equation is balanced by adjusting the coefficients (numbers in front of chemical formulas) to ensure that the number of atoms of each element is equal in the reactants and products.
Chemical equation that is and its parts
The equations are palpable samples of a series of transformations or changes in natural chemical processes of matter that are generated around us. Modifying the properties and characteristics of the initial reactants in terms of substances with atoms, with different nature and names.
It should contain all the information that describes the entire process. Taking into account the above, the way to write a chemical equation will be. First, the material or materials to be transformed, which are called reactants, is placed on the right side joined with a plus sign (+).
Followed by an arrow, which means the activity or dynamism of the process, it can be a single or several, is placed (+). That is, the transformation. Subsequently, the materials that originated are written, which are named as products. The chemical symbols of the elements are used for both the reactants and the resulting ones.
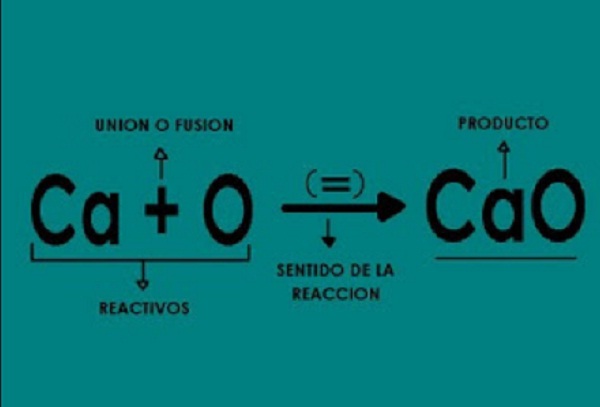

In addition
The aggregation status of the element is also placed in parentheses with lowercase letters. In order for the chemical equation to be represented correctly, the following must be fulfilled. Chemical symbols are written from the right side and from the left side, after the arrow they should be corresponding to the matter participating in the reaction.
The equations must be balanced, using integers called coefficients, be concordant between the reactant materials and the products in terms of mass and charge.
Chemical exchange rates
The innumerable changes that occur in natural processes happen in four ways, the combination, where two or more materials are joined to produce one with only complex characteristics with respect to the initial ones. Displacement is the substitution of one element for another within a chemical compound.
Decomposition, the rupture of one material occurs, into another of a simple nature, such as elements or chemical groups. The double decomposition, the separation of the components of some materials to form others different from the initial ones. It is also important to note that the equation uses some conditions that also have symbols.
Which are written above the arrow as they indicate a definite action. With a triangle above the dotted arrow, presence of heat. On a segmented arrow put above a line with four vertices, presence of electricity. Presence of catalyst, a dotted arrow. An arrow pointing down, formation of a precipitate.
The release of a gas, an arrow pointing upwards.


Examples of a chemical equation.
For the combination reactions corresponds a total of 6
exchange rates are:
1.- A metal plus oxygen to produce a basic oxide
Hg+2 + O2
- The HgO
2.- No more metal an oxygen producing an acidic oxide or
anhydride
N2 + O2
- Oxygen 3/2N2O3
3.- The basic oxide plus water to give a hydroxide
CaO + H2O
- Ac voltage(OH)2
4.- Anhydride plus water results in an oxacid acid
P2O3 + 3H20 -1
2H3(PO3)
5.- Metal plus a non-metal you get a salt
Br2+
2Na - to 2NaBr
6.- A metal bonded to a hydrogen results in a hydride
H+ + Cl-
- With HCl
For displacement reactions where a
element on the other hand, within a compound
chemical. Which is represented as a metal bonded to an acid producing
a salt plus hydrogen.
Zn +
H2SO4 -1
Zn(SO4) + H2 i
For decomposition reactions, a rupture occurs or
separation of the components of a compound, occasioned by the supply of
heat or electricity.
KClO3(g) —1
KCl + 3/2O2(g)
For a double decomposition reaction, it encompasses the rupture
of chemical species, followed by a rearrangement of the components.
BaO + 2HCl —1
BaCl2 + H2O
ZnO + 2HNO2
-- Zn (NO2)2 + 2H20
Balancing chemical equations
To start the balancing it is imperative that the chemical formulas of the materials involved are correctly written. The word balance is synonymous with balance, moving from one side to the other until the materials are stabilized. It is to equalize the charges and masses of the reactants with the products in order to fulfill the law of conservation of masses.
In order for a chemical reaction to swing, it must be considered: Identify the reactants and the resulting substances. Verify that it is well written and complete. Let the subscripts indicate the number of the appropriate atoms. Stoichiometric coefficients affect all present matter.
How to perform the balancing to a chemical equation?
It should be started by the metallic element followed by non-metallic (other than oxygen or hydrogen). These were adjusted at the end, as has been mentioned because they are incorporated in the water. Use small numbers and integers and incorporate them as coefficients in the middle of them, except for one as it is understood.
You cannot divide the formulas to place the coefficient or change the subscript numbers. The number of atoms are counted by multiplying the coefficient by the subscripts of the formulas. Adding up all the atoms of an element on the same side of the equation. Do this summation under the elements on each side of the equation.
I hope this article has been very useful to you, check this material every time you need it.

