- Definition of titration
- What is titration in chemistry?
- Procedure of titration:
- Steps involved in titration:
- How Does Titration Work?
- Titration Apparatus
- Types of Titration
- Advantages and Disadvantages of Titration
- Safety Considerations
- Step-by-Step Process of Titration
- Steps to Do Titration Problems
- Safety Precautions for Titration
- Equipment Required for Titration
- Calibration of Equipment Required for Titration
- Conclusion
A Titration is a method utilized in chemistry to identify the concentration of a reactant in a solution that is unknown. At the same time, a known solution is included in an unidentified solution till a reaction takes place. Typically, this reaction entails a color change.
A titration will certainly produce very accurate results for different sorts of titrations, such as acid-base, redox complexation, and precipitation reactions when it is carried out properly as well as meticulously.
Definition of titration
Titration is a laboratory technique commonly used in analysis to determine the concentration of an unknown substance. It involves carefully adding a known volume of a reagent of known concentration to an unknown solution until an endpoint or equivalence point is reached. This endpoint can be determined either volumetrically or electrometrically. Titration is used for different purposes in the laboratory: assaying for concentration, determining acid-base equilibria, and performing qualification and quantification of components in samples.
What is titration in chemistry?

A titration is specified as" it is a process of determining the concentration of a sample (analyte) by adding known increments of substance (titrant) with which it responds till exact chemical equivalence is accomplished (the equivalence factor)".
Titration is an analytical method that enables us to identify the concentration of an unknown analyte by adding a titrant solution with a recognized concentration. The analyte as well as titrant respond according to a recognized stoichiometric relationship, such that the reaction will eat all analytes at some point throughout the addition of the titrant.
Therefore, the volume and molar stoichiometry of the titrant were included in permit the determination of the unknown concentration. There are a selection of indicators that can be utilized to determine the endpoint of the titration. The choice of the indicator is dependent on the indicator's acid strength.
Preparation of 0.1 n sodium hydroxide (NaOH) and also its standardization with standard hydrochloric acid (HCl) solution is one example of acid-base titration.
Procedure of titration:
In the titration procedure, a titrant is prepared, which is a standard solution whose volume as well as concentration are predetermined. After that, the titrant is blended with the analyte up until a particular endpoint or equivalence factor is reached.
At this stage, the quantity of titrant consumed can be used to compute the concentration of the analyte. Alternatively, titration is an idea of stoichiometry utilized to establish the concentration of a solution whose value is unknown.


In terms of steps of the treatment, the exact amount of analyte is absorbed the conelike flask. A few drops of the indicator are after that placed underneath the calibrated burette that holds the titrant. The titrant is added dropwise into the analyte as well as the indicator in small volumes.
This will certainly proceed until the indicator reacts to the titrant saturation threshold as well as adjustments color. At this moment, this would certainly show that comes to the endpoint of the titration. In this situation, the amount of titrant balances the quantity of analyte existing throughout the reaction.
Consequently, the volume and molar stoichiometry of the titrant were added to allow the determination of the unknown concentration. There are a selection of indicators that can be utilized to establish the endpoint of the titration. The choice of the indicator depends on the indicator's acid strength.
Preparation of 0.1 n sodium hydroxide (NaOH) and its standardization with standard hydrochloric acid (HCl) solution is one instance of acid-base titration.
Steps involved in titration:
- Before beginning, gather all the required apparatus such as conical flask, burette, burette, stand, pipette, beaker, volumetric flask, funnel, etc.
- Glassware should all be washed, rinsed, and properly dried according to standard laboratory procedures.
- Before filling the burette for the titration, rinse it with distilled water and then pre-rinse it with a portion of the titrant solution. Pre-rinsing is required to make sure that all solution in the burette is the desired solution, not a contaminated or diluted solution.
- Fill the burette with an excess amount of prepared titrant.
- Carefully clamp the burette to a burette-stand.
- Remove the air bubbles by tapping the burette or by draining some volume of titrant and adjusting it to zero reading.
- Measure precisely the amount of analyte to be used and pour it into a flask.
- Then add a few drops of indicator as per the procedure into the flask.
- If required, add a second chemical.
- Put the flask in place beneath the burette.
- Slowly rotate the stopcock to the open position to allow the titrant drips out of the burette.
- Once the reaction is completed (Equivalence/endpoint is reached), properly record the burette reading.
- It is recommended that to get accurate results, repeat the titration three times.
- Dispose of used chemicals in a clearly labeled waste container.
- Effectively clean glassware by rinsing it with water after use.
- Take their mean and calculate the molarity/normality/concentration of the sample.
How Does Titration Work?
The basic process of titration involves mixing a sample solution of the unknown substance with a reagent of known concentration in a titration apparatus, up to a certain point where they react. By measuring the precise amount of reactant that has been added, the precise concentration of the unknown substance can be determined. The most common titration involves acid-base reactions, although there are several other reactions that can take place, such as redox, complexometric, and precipitation.
Titration Apparatus
Titration requires specific equipment to be completed. A titration apparatus usually consists of two compartments, a burette and a Erlenmeyer flask. The burette is a glass tube filled with the known reagent, while the Erlenmeyer flask contains the sample solution. The burette is connected to a stopcock (or tap) which allows for precise amounts of the reagent to flow into the flask. The flask also contains an indicator, which changes color when the reagent and the sample have fully reacted.
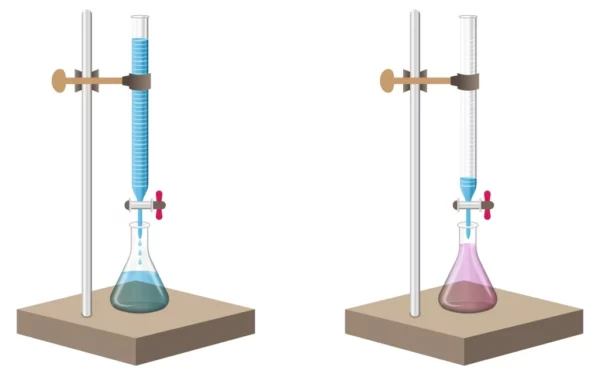

Types of Titration
Titrations can be classified into three main categories: acid-base, redox (oxidation-reduction), and complexation titrations.
Acid-Base Titrations
In this type of titration, a standard acid is titrated with a solution of a strong base, or vice versa. An indicator is used to identify when the end point has been reached, which occurs when the acid and base react to form a salt and water.
Redox Titrations
Also known as oxidation-reduction titrations, these involve adding a reagent into the sample solution which reacts with the analyte until it reaches an oxidized or reduced state. This type of titration is used to measure the amount of an oxidizable or reducible species present in the sample. A redox indicator is used to show the end-point of the titration; this is usually a colour change from the original colour to a new colour.
Complexation Titrations
In this type of titration, a sample is titrated with a complexing agent in order to determine the amount of a given species present in the sample. A complexing agent is a substance which forms a complex with the analyte, and this type of titration is used to measure the amount of the analyte present. A change in colour of an indicator is used to detect when the end-point has been reached.
Advantages and Disadvantages of Titration
Titration has several advantages, such as its accuracy, cost effectiveness, and the fact that it can be used to measure a wide range of concentrations. It is also relatively simple to set up, which makes it ideal for students who are just starting to experiment with titrations in the laboratory.
However, titration has some drawbacks. Firstly, it is time consuming and requires patience, as it can take several minutes to complete a titration. Secondly, the accuracy of the results can be affected by external factors such as the temperature of the reactants and the kind of indicator used. Finally, precise measurements and equipment are required in order to obtain accurate results.
Safety Considerations

Safety is an important factor when working with titration measurements. Any lab worker handling the titrant and the unknown should be wearing proper safety equipment such as lab coats and safety glasses. Depending on the titrant and unknown, it is also important to use appropriate containers for storage and disposal. Additionally, the work area should be well ventilated and all potential hazards should be identified, monitored, and reported.
Step-by-Step Process of Titration
Although there are many different types of titrations, all require the same basic steps for successful completion. Here is a step-by-step guide to the titration process:
Step 1: Prepare the Titrant
The first step in performing a titration is to accurately measure and prepare the titrant, the reagent of known concentration which will be used to determine the concentration of the unknown analyte. Accuracy is of the utmost importance here, as any errors in measuring or preparing the titrant can lead to erroneous results.
Step 2: Prepare the Sample
Once the titrant has been prepared, the sample containing the unknown analyte should be prepared. This often involves weighing and diluting the sample, based on its composition and the type of titration being performed.
Step 3: Dispense the Titrant into the Sample
The third step in the titration process is to dispense the prepared titrant into the sample. This is done slowly and accurately, using a burette or other dispensing device.
Step 4: Monitor the Reaction
Once the titrant has been added to the sample, it is important to monitor the reaction carefully. Depending on the type of titration being performed, this may involve the use of a pH indicator (for acid/base titrations), a calorimeter (for redox titrations), or other means.
Step 5: Calculate the Concentration of the Analyte
The final step in a titration is to use the measured reaction to calculate the concentration of the unknown analyte. This is usually done by calculating the molar concentration of the analyte from the concentration of the titrant and the volume added.
Steps to Do Titration Problems
Titration problems can be solved by following the following steps:
Step 1: Calculate the Molarity of the Titrant
The first step is to calculate the molarity (M) of the titrant, that is, the concentration of the titrant in terms of moles of solute per liter of solution. This is usually given on the label of the titrant solution.
Step 2: Calculate the Volume of Titrant Needed
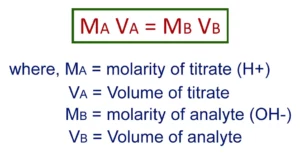

The next step is to calculate the volume of titrant that is needed to completely react with the amount of analyte present. This is done by using the formula: Volume of Titrant (V) = Moles of Analyte (n) / Molarity of Titrant (C).
Step 3: Choose an Indicator
The third step is to choose an indicator. This is necessary because the endpoint of the titration must be accurately identified. The indicator should be selected so that it changes colour at the equivalence point.
Step 4: Perform the Titration
The fourth step is to perform the titration. This is done by slowly adding the titrant to the analyte, using a burette, until the endpoint of the titration is reached. The endpoint is the point where the indicator has changed color, signifying that the titration is complete.
Step 5: Calculate the Concentration of the Unknown Solution
Once the endpoint has been reached, the concentration of the unknown solution can be calculated using the formula: Concentration of Analyte (C) = Volume of Titrant (V) X Molarity of Titrant (M).
Safety Precautions for Titration
It is important to take some safety precautions before performing a titration. Safety glasses and lab coats should be worn when handling any chemicals or reagents. Any potential hazards should be clearly identified, and appropriate safety measures should be taken. Gloves and eye protection should be worn when handling any concentrated acids or alkalis. Additionally, titrations involving heat should be conducted in a fume hood to prevent exposure to hazardous fumes.
Equipment Required for Titration
The equipment necessary for titration will vary depending on the type of titration being performed. The most common types of titrations are acid-base titrations and redox titrations. For these types of titrations, a burette, a pipette, and an Erlenmeyer flask are required for the sample and titrant solutions. An analytical balance, an thermometer, and an indicator are also necessary for monitoring the reaction.
Calibration of Equipment Required for Titration
Before performing a titration, the necessary equipment should be calibrated. This means checking the accuracy of each piece of equipment and ensuring that it is within the specifications for the given titration. The burette and pipette should be checked for accurate measurements, and the analytical balance should be calibrated in accordance with the manufacturer's instructions.
Conclusion
Titration is a useful analytical tool that can be used to determine the precise concentration of a substance. By slowly adding a reagent to a known solution and measuring the amount of reactant added, the precise concentration of the unknown substance can be determined. This process is useful in a variety of fields and can be used to measure concentrations of acids, bases, and other types of compounds. However, it can be time consuming and requires precise measurements and equipment in order to obtain accurate results.
Recomended videos
References
A. Wikipedia: https://en.wikipedia.org
B. Knauer: https://www.knauer.net
C. Kromasil: https://www.kromasil.com
D. Shimadzu: https://www.shimadzu.com
E. ChemistryView: https://www.chemistryviews.org

