Isocratic vs Gradient
There are two different ways by which we can run the HPLC method namely isocratic elution(isocratic hplc) and gradient elution(hplc gradient). In isocratic elution, a mixture of mobile phase or a solvent system used to separate the sample components and it is consistent over the complete testing time. There are no changes in the mobile phase composition that can be made between the entire run of the isocratic system.
The composition of the mobile phase in the gradient system varies throughout the chromatographic run and therefore affects the retention of the analysis. Separation in gradient mode can be either accelerated or decelerated. In the gradient system, the composition of the mobile phase should be changed concerning time so it is a flow gradient if the mobile phase ratio is not changed throughout the process of analytes separation then it is considered as isocratic elution.
The key difference between isocratic(I) and gradient(G) systems is that the isocratic elution uses a single mobile phase composition having the same polarity, whereas the gradient elution uses more than one mobile phase and it can gradually increase or decreases the polarity of the mobile throughout the process of separation.
The key distinction between I and G elution is that isocratic elution is concerned with sustaining a recurring concentration in the mobile stage, whereas gradient elution is concerned with sustaining a variable concentration in the mobile stage.
The terms isocratic and gradient elution are used in chromatography. In the course of a chromatographic development, we use a stationary stage, which is a non-mobile substance, coupled with a mobile stage, the mobile substance. The isocratic and gradient elution details the characteristics of the mobile stage.
Define Elution - What is Elution
Elution is the process of extracting one material from another by washing with a solvent
Define Elute
The elute is the analyte material that emerges from the chromatograph. It specifically includes both the analytes and solutes passing through the column, while the eluent is only the carrier.
Define Eluent
The eluent or eluant is the "carrier" portion of the mobile phase. It moves the analytes through the chromatograph. In liquid chromatography, the eluent is the liquid solvent; in gas chromatography, it is the carrier gas.
What is isocratic elution? (isocratic hplc)
Isocratically meaning example
Samples were loaded onto the gel filtration column and eluted isocratically in 100 mM sodium phosphate + 100 mM NaCl buffer pH 7.2 at a flow rate of 0.5 ml min
Isocratic elution is a term used in chromatography when the mobile stage has a recurring concentration. Here, the concentration of the mobile stage is recurrent throughout the entire chromatographic run. In this development, we have the possibility to see that the peak width increases with retention time in a linear fashion in the chromatogram. However, this brings a disadvantage: the late eluting peaks become very flat and broad. Then, these broad peaks become difficult to admit as peaks.
Furthermore, in isocratic elution(IE), the selectivity does not change in functionality of the column dimensions. This means that selectivity is not dependent on changes in column dimensions. Here, length and diameter are thought of as column dimensions. Then, the peaks elute in the same order.
What is gradient elution? (hplc gradient)
Step elution is a term used in chromatography when the mobile stage has a variable concentration. In other words, the concentration of the mobile step does not have to be kept recurrent. Among other things, in HPLC, a common splitting procedure uses 10% methanol at the beginning and ends at 90%, with the concentration gradually increasing. The mobile stage has two components: a weak solvent and a strong solvent. The weak solvent makes it easier for the solute to elute slowly, while the strong solvent makes the solute elute slightly. In reverse-stage chromatography, we use water as the weak solvent and organic solvent as the strong solvent.
In addition, the gradient elution procedure decreases the later eluting elements to make them elute more rapidly, giving a tight peak in the chromatogram. This procedure optimizes the shape and height of the peaks. Furthermore, in the gradient elution technique, the order of elution changes with changes in column dimensions.
What is the difference between isocratic vs gradient elution?
The terms isocratic and gradient elution are used in chromatography. Isocratic elution and gradient elution describe the characteristics of the mobile stage. The key distinction between isocratic and gradient elution is that IE refers to the maintenance of a recurring concentration in the mobile stage, whereas gradient elution refers to the maintenance of a varying concentration in the mobile stage.
In the isocratic elution technique, the peak width increases with retention time in a linear fashion. However, in the gradient elution technique, the retention of the later eluting elements decreases, whereby the elution becomes efficient and gives narrow peaks. Furthermore, in isocratic elution, selectivity is not dependent on column dimensions, but in gradient elution, selectivity changes with changing column dimensions.
The following table summarizes the distinction between IE and GE.
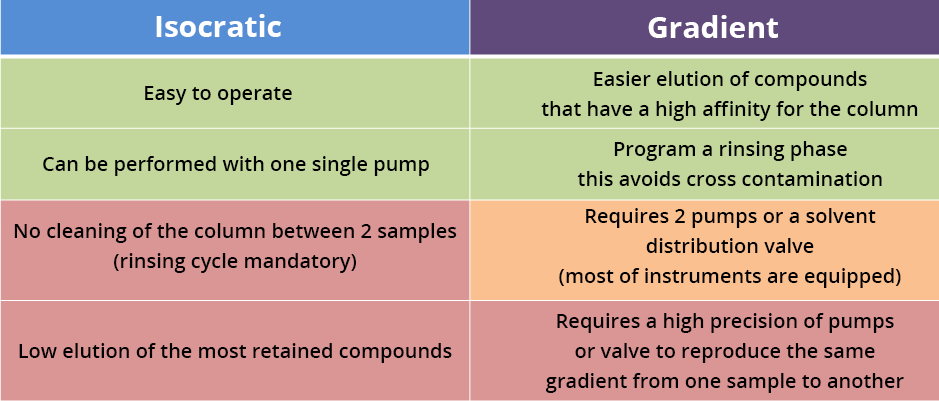

Summary - Isocratic elution versus gradient elution
Isocratic elution and gradient elution describe the characteristics of the mobile stage. The key distinction between isocratic and gradient elution is that isocratic elution refers to the maintenance of a recurring concentration in the mobile stage, whereas gradient elution refers to the maintenance of a varying concentration in the mobile stage.
Reference:
- "IUPAC Gold Book: eluent". International Union of Pure and Applied Chemistry. doi:10.1351/goldbook.E02040. Retrieved 2008-09-28.
- George H. Roberts (2006). "Elution Techniques in Blood Bank" (PDF). American Medical Technologists (AMT).
- “Gradient Elution.” Gradient Elution – an Overview | ScienceDirect Topics, Available here.
- Elution on Wikipedia Available here.
- Brown, Phillis (2001). Advances in chromatography. CRC Press. p. 36. ISBN 0-8247-0509-2.
Image Courtesy:
1. “HPLC extraction and use” By USDA – Flickr (Public Domain) via Commons Wikimedia