¿Hplc Column? All about this chromatography advance!



Hplc Column are the main component in HPLC because is responsible for the separation of the sample through with the mobile phase and separates in its components when it comes out from the column.
Different types of HPLC column are used in analysis of different pharmaceutical compounds according to their nature and column separatio capacity.
Generally, silica gel is filled in the high-performance liquid chromatography columns because of its particle size and of components and silica gel is also an inert material that does not react with mobile phases. Therefore silica colu compounds of different chemical natures. The material filled in the HPLC columns is known as a stationary phase. There are different types of chromatography columns on the basis of their composition and method of separation.
So...What is hplc column !
Normal Phase Columns // Reverse Phase Columns // Ion Exchange Columns // Size Exclusion Columns
Normal Phase HPLC Columns: This type of columns has more polar stationary phase than the mobile phase. The packing material of the colum mobile phase and this condition is fulfilled by the silica that is polar material. But water is more polar than the silica, methylene chloride, hexane and chloroform or a mixture of these with diethyl ether is used as mobile phase. Separation of the sample components occurs on the basis of the polarity of the sample components. Sample c interact more with polar stationary phase resulting in separation from the less polar component that interacts with columns are widely used in the pharmaceutical analysis. The chromatography column packing in which normal phas Normal Phase Chromatography.


- Reverse Phase HPLC Columns: In reverse phase columns as its name states, it is reverse of the normal phase columns. It has a non-polar or less more polar mobile phase. Bonded hydrocarbons like C8 and C18 and other non-polar hydrocarbons are used as s columns while aqueous organic solution like water-methanol or water-acetonitrile mixture is used as mobile phase. Separation of sample components in reverse phase columns also occurs on the basis on the polarity of the sample opposite of the normal phase HPLC columns, therefore, this type of chromatography is known as Reverse Phase C
Ion Exchange HPLC Columns: The compounds those can easily ionize are analyzed using these columns. Stationary phase in these columns negative or positive charge while mobile phase is a polar liquid as the salt solution in water. Separation of mole attractive ionic force between molecules and the charged stationary phase. Due to the exchange of ions d components, it is known as Ion Exchange Chromatography.
HPLC columns have a different length varying from 30 mm to 250 mm and their particle size or porosity from 3 analysis of sample, therefore, these are considered important during the HPLC analytical method development. Co the nature of the compound to be analyzed and the mobile phase. Column performance should also be evaluated runs or as required.
HPLC stands for High-Performance Liquid Chromatography, formerly referred to as High-Pressure Liquid Chromatography.
Types of HPLC columns
To classify liquid chromatography, there are several ways. If this classification relies upon nature of the separation process and the stationary phase, then it can be adsorption chromatography, ion-exchange chromatography, and size exclusion chromatography. In relation to the first type, two modes are defined relies on polarity to two phases, i.e. normal phase and reverse-phase chromatography, as mentioned types about 90% cover of all chromatography applications and for the separation of components the column are used.
Columns are usually made of SS, around 50 and 300mm in length and within 2 and 5 mm, they are typically packed with a stationary phase of 3 to 10 µm size. Columns of the internal distance of less than 2 mm are acceptable regularly in the form of microbore HPLC columns.
Types of packed gels:
Silica gel:
Silica gel is the most popular packing material used. Silica gels are available in two types it is a spherical and irregular shape, Spherically shaped gels are most commonly used. There is a pore on the surface of the silica gel used in liquid chromatography. By contains the pores, it gives a bigger surface area than without holes. The most silica columns nowadays have spherical types and small size particles have been developed. The 5μm is a most commonly used size, but small size 1.5 to 3μm gel is also used in the HPLC column. Small gels are packed in small column housing and therefore retention time is reduced.
Polymer gel:
Silica gels were always used in the earlier stage of HPLC development. But, the polymer-based column is getting well-liked. Polyethylene and polypropylene are generally known polymers. Like silica gel, polymer gel columns are available in very small particles. Polystylene, Polymethacrylate, polyhydroxymethacrylate, Polyvinyl alcohol are types of polymers.
Other gel:
In addition to silica and polymer gels, the gels used are natural substances like Cellulose, chitosan agroceae, and dextrin and ceramics for example zirconia and hydroxyptite are used in liquid chromatography for the separation of analytes but is have very limited use.
There are several types of columns used in the separation of components with the help of the mobile phase, which is based on the separation mode used in liquid chromatography. Here some types of separation modes are mentioned.
HPLC column
The most HPLC columns are made from stainless steel, the actual separation happen into the column usually columns are 30 to 250 mm in length, 01 to 05 mm in diameter and 03, 05 and 10 microns in pore size. The column is packed with porous particles. The Porous particles are made from polymer and surrounded by a thin equal layer of silica and polystyrene.
In early years LC carried out in a glass column with diameter 01 to 05 cm and length 50 to 500 cm. Commonly HPLC have guard column in front of the analytical column to protect from contaminants and remove particulate material it helps to extend the life of analytical column, the guard column and analytical column have same stationary phase.Here are listed some common bonded phase for HPLC columns.SI, C1, C2, C3, C4, C5, C6, C8, C18, CN, NH2, NO2, OH, PHENYL, SCX, SAX, WCX, WAX.
HPLC column loading capacity
The column loading depends on what sample is, how clean it is or how dirty/impurities it is, how soluble it is insolvent and the mobile phase. When we are performing a separation of analytes in HPLC, the column should not be overloaded because the response will be affected. As a common rule, do not load more than 5% of the column volume. Volume overload and mass overload are the types of overload in chromatography.
In mass overload, excess amount of analyte is injected and in volume overload excess amount of liquid is injected onto the column. The peak symmetry of analyte in high-performance liquid chromatography depends on the mobile phase composition, the column or stationary phase and the sample volume used. If the column is overloaded it will affect peak shape and peak height by increasing the peak broadening, tailing, and width.
The maximum injection volume in a column depends on several parameters such as.
- The type of detector has been used in it as it will affect the sensitivity issue.
- Column dimension i.e. particle size, internal diameter, and length.
- Type of the column used to separate the components.
- Once the analytical method is optimized, to determine the capacity of the particular packing material a loading study is performed on the analytical column.
Find out how much mass we need to separate the components. Once the required mass is recognized, several simple equations can be used to calculate the size of the column required for purification. As well, the preparative HPLC system needs to consider the maximum flow rate and backpressure and may limit the column size.
HPLC column care and maintenance
The column is a key factor in HPLC chromatography separation hence preventive care and maintenance of HPLC column required for better performance and increasing span life of a column. In HPLC analysis every chromatographer know the importance of column, because of the theoretical plate number, resolution, tailing factor, peak symmetry, and system suitability depend on the column performance. 90% of analysts work in HPLC chromatography carried out by the reverse phase chromatography, therefore, some important points consider that to protect and helpful in growing the life and performance of the HPLC column are mentioned here.
1. Install the column appropriately when you replace or change it.
2. Confirm the performance of new column supplied by the vendor.
3. Always use guard column to protect from impurities and other foreign particulates.
4. Protect the column from bumps, knocks and mechanical shock.
5. Always use HPLC grade solvents and water for analysis.
6. Always use fresh water and buffer for analysis.
7. To prevent impurities and foreign particles, filter the mobile phase by using 0 .45 μ filter
8. Filter the sample using 0 .2 μ filter before injection.
9. Degas all mobile phase and samples.
10. Periodically check the column performance.
11. Keep the back pressure below 4000 psi.
12. Avoid making changes in any sudden pressure.
13. Always saturate the column with the mobile phase before sampling.
14. Maintain the temperature of the column oven if available.
15. The pH of the mobile phase or buffer should be in between 3.0 to 7.5 pH.
16. Avoid use of phosphate buffer more than 20 mM.
17. Avoid using a viscous buffer.
18. Do not overload the column.
19. Avoid injecting biological samples directly into HPLC injectors.
20. Avoid injecting of high acidic or basic sample directly in the sample loop.
21. Avoid store the column with buffer.
22. Never let the column dry, it should be wet with the appropriate solvent
23. Avoid working with high flow rates, it creates pressure
24. Wash the columns properly after every use with HPLC grade water and methanol/acetonitrile.
25. Store the column in a solvent like methanol/acetonitrile.
To improve column performance, follow the above points in your regular practice.
Effect of pH on HPLC columns
The proper use of HPLC columns is of utmost significance for the life span of a column. Generally, columns of reversed-phase chromatography is stable within a pH range of 2 to 8. If you determine a pH value, the measurement should be performed before mixing with organic solvents in aqueous media. Nowadays HPLC columns are available to use outside that pH range. However, if the pH range of the mobile phase is outside the pH range of 2 to 8, ensure the seller's product information before using silica-based columns.
As the pH of the mobile phase/buffer/sample is considered as a parameter in RP-HPLC, not only its effects on retention time but also the variation in asymmetry and efficiency of the chromatograph need to be considered. The stationary phase (column) is affected by pH. At very basic pH i.e. below pH 02.00, the bonded stationary phase will be stripped of the silica support. Silica at high pH i.e. above pH 08.00 will be damaged by self-dissolution.
The separation of basic molecules at low pH is often recommended in RP-HPLC since symmetric peak shape and maximum column efficiency are usually the result. However, analysis at low pH (below pH-3) is not possible due to of instability of solute or band-spacing issues. In such cases, the pH between 04.00 to 08.00 or higher is used to separate. Even though working on the intermediate pH can generate useful band spacing for ionizable molecules, difficulties with retention reproducibility and band size may occur as a result of partial ionization of the basic molecules.
¿Why is silica polar in HPLC columns?
The surface of silica (glass and sand are essentially all silica) is polar because it is covered with silanols (Si-OH). The -OH is a polar functional group (think alcohols), and serves as both a Hydrogen-bond acceptor and donor. Thus, there are many possible interactions with alcohols, amines, acids, and carbonyl compounds. Also, silanols are acidic, having a pKa about about 4.5 (similar to many weak organic acids). So, at a pH above about 5, the surface of silica has a negative charge. At a pH above 7, the silica starts to dissolve. All of these things contribute to silica being considered “polar.”
When bare silica is using in LC, these polar interactions occur with the compounds being separated and the mobile phase. When using less polar solvents like hexane, this is call “normal phase chromatography.” When used with a polar mobile phase like water and acetonitrile, the operating mode is known as aqueous normal phase or hydrophilic liquid chromatography (HILIC).
If you react the silanols and replace them with a chain of carbon atoms (e.g., C18), the surface loses its polarity and is now hydrophobic. When used with a more polar mobile phase, this is called “reversed phase chromatography.”
Hplc column Silica
Silica for HPLC Column
HPLC Column chromatography is the ideal method of chromatography for purification and separation. It is a technique in which the stationary phase is solid adsorbents like silica gel and activated alumina powder and the mobile phase is a liquid.


The principle of active compound separation depends on the activity of adsorbents and polarity of the solvent. If the polarity of the solvent is very low and the activity of the adsorbent is strong and high, then result of separation of compound is good. On the other hand, if the polarity of the solvent is very high and the activity of adsorbents is high then it gives poor results of compound separation. It means purification and isolation of compounds are not 100% pure. The process of column chromatography is the oldest and the most common technique f or the separation of complex mixtures packed in a column.
Chromatography is a technology by which a mixture of chemicals are separated by its components between two phases like stationary phase which is remain fixed in placed using two adsorbents such as silica gel and activated alumina, while as mobile phase is another method which is slowly movable and flows down through the column by either gravitational forces or external pressure into the column. The Stationery phase may be solid or liquid and the mobile phase is always in solid liquid foam use different solvents.
Analytical Chromatography: The collectively term of chromatography is may be analytical or preparative. The starting phase of chromatography is analytical chromatography with little amount of silica gel mesh 60-120 size by using analytical column packaging, to analysis how many percentage of mixture is purify. Analytical chromatography is a simple method of chromatography with faster and cost effective separation. In analytical chemistry development, techniques for solving chemical subtracts by using thin layer plates coated silica gel on glass plate. This technique becomes standard analytical tools in pharmaceutical laboratories.

Preparative Chromatography: Focus on the latest chromatography technologies such as preparative and process chromatography to optimize the current and standard opportunities to optimize chromatography process in proper way. After preparative process, improving impurity profile and speed of separation use semi-preparative chromatography.
Process Chromatography: Process chromatography technology is utilized on a large scale with dynamic binding capacity. We are developing large scale process chromatography for improving purity in various biologics such as proteins, virus, vitamins, hormones, anti-bodies. In process chromatography use stationery phase adsorbents like silica gel and activated alumina are used in bulk quantity. Silica gel 230-400 and 400-800 mesh sizes are used in process chromatography to get more purity of active compounds.
silica column hplc
Gravity Chromatography: Gravity chromatography is a manual process of require continuously observed. Solvent allowed to move down the column by gravitational forced and the flow rate can be manually controlled. The practical size use of silica gel is 70-230 mesh and 63-210 microns.
Hplc Column Volume Calculator
The following equations are for the novice chromatographer. More advanced calculations can be found on the system suitability and pressure-flow pages.
1. Calculation of total column volume (also used for calculating bed volume)
2. Calculation of empty column linear flow rate from volumetric flow rate
3. Calculation of volumetric flow rate from empty column linear flow rate


1. Calculation of total column volume (also used for calculating bed volume):
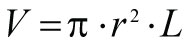
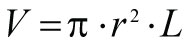
Where:
V = calculated column volume
r = column radius
L = length of the column (or packed bed)
2. Calculation of empty column linear flow rate from volumetric flow rate:
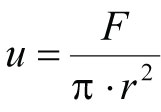
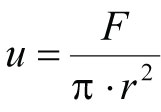
Where:
u = Linear flow rate (cm/hr)
F = volumetric flow rate (mL/hr) [multiply mL/min x 60 min/hr]
r = column radius (cm)
3. Calculation of volumetric flow rate from empty column linear flow rate
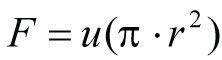
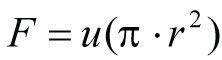
Where:
F = volumetric flow rate (mL/hr) [divide by 60 min/hr to get mL/min]
u = Linear flow rate (cm/hr)
r = column radius (cm)
The term column volume, typically abbreviated CV, is a value used to help determine separation quality and loading capacity. But how can this concept and its definition be better understood? Bob Bickler explains it all on the Flash Purification Blog.
Some chemists think the internal volume of the cartridge without packing material inside is the column volume. While useful in determining scale-up factors, the empty column’s volume is not the CV. The CV of any column or cartridge is the volume inside of a packed cartridge not occupied by the media. This volume includes both the interstitial volume (volume outside of the particles) and the media’s own internal porosity (pore volume). Combined, the two volumes constitute 70% to 80% of the packed cartridge’s volume. Of course this means that the media only occupies 20% to 30% of the space in the cartridge.
Hplc column volume calculator
¿How to select a column for HPLC method development?
In high-performance liquid chromatography, to separate the components is our object and this occurs in the column (Stationary Phase), hence the column is the heart of the HPLC system. Changing the HPLC columns during method development will have the most impact on the resolution of the analytes.
Typically, current reverse phase chromatography columns are made by packing with globular silica gel beads that are coated with the hydrophobic stationary phase. Typically the nature of the stationary phase has the most influence on the elution, capacity factor, selectivity, and efficiency. There are various types of matrices for stationary phase support, including polymers, silica, and alumina.Silica is the most regular matrix for HPLC columns. Silica is chemically stable for low pH systems and most organic solvents. The drawback of silica solid support is that it will dissolve above pH 7. Nowadays HPLC columns are developed for use in high pH range. The particle size, nature, and shape of silica effect the separation of analytes.
The use of small particle size of silica increases the separation efficiency or increases the number of theoretical plates. But, the use of small particles increases the backpressure of the system and the column becomes more easily plugged. The mobile phase in RP-HPLC is polar and the stationary phase is non-polar, whereby polar molecules are usually eluted earlier than non-polar molecules.
To form a stationary phase for RP-HPLC on silica supports, to introduce a non-polar surface free silanols are reacted with a chlorosilane with hydrophobic functionality. Because of static barriers, only about 1/3 of the silanols are derivatized. The remaining silanols may interact with the molecules, resulting in peak tailing. Typically, after column derivatization with the preferred stationary phase, chlorotrimethylsilane reacts with column STEM to eliminate the remaining free silanols and improves the efficiency of the column. C18 (octadecyl), C4 (butyl), Cs (octyl), phenyl (phenyl propyl) and nitrile (cyanopropyl) column are commonly used stationary phases.
In common, higher carbon loads higher phase loadings, and longer alkyl chains gives better retention of non-polar components.Here are listed some common bonded phases for HPLC columns.SI, C1, C2, C3, C4, C5, C6, C8, C18, CN, NH2, NO2, OH, PHENYL, SCX, SAX, WCX, WAX.
Factors affecting column efficiency in HPLC
The column or stationary phase selection is the most significant advance in analytical method development. Without a column, which is stable and high performance, the development of a reproducible and rugged method is not possible. The selection of the column is done based on information about the nature and analysis of solutes.
Usually, a lengthy column gives improved separation because of higher theoretical plate numbers. A column with a 5-µm particle size provides good reproducibility, efficiency, and reliability.The column efficiency is reported as the number of theoretical plates. The efficiency of the column in HPLC is dependent on various factors; some factors that affect column efficiency in HPLC are given below.
- The activity of the adsorbent
- Column length
- The dimension of the column
- The flow rate of the system
- Packing of the column
- Particle diameter
- Particle size distribution
- Quality of solvents
- The temperature of the column
Difference between C8 and C18 column in HPLC
High-performance liquid chromatography is a method used to separate, identify, and quantity of each analyte in the complex mixture using a mobile phase. Both C8 and C18 refer to the bonded face of the alkyl chain and both are used in HPLC separation.
There are numerous different kinds of reverse phases there in the market, including C8 and C18 columns. C18 is the most popular one than a C8 column. C8 and C18 are both reversed-phase columns, both columns refer to the alkyl chain length of the bonded phase. The length of the chain affects the hydrophobicity of the sorbent phase and therefore increases the retention time of the component. C18 have the maximum amount of hydrophobicity, since the longer length of the carbon chain, C-18 is extra hydrophobic compared to the reverse phases.
C8 column is used while small RT is desired, if hydrophobicity is low, there is less retention time for non-polar analytes, therefore, the non-polar analytes or compounds separate out more quickly with C8 column. The C8 is select over the C18, in the reverse phase matrix where the degree of hydrophobicity is low. But, the C18 column is more accepted and broadly used because C18 silica gel interacts with the broad range of analytes, hence it used in the separation, qualitative and quantitative studies in the pharmaceutical industries, chemical analysis, and environmental science.
- C18: Octadecyl silane
- C8: Octyl silane
- In reverse phase HPLC: the mobile phase is polar and the stationary phase is non-polar.
- In normal phase HPLC: the mobile phase is non-polar and the stationary phase is polar.
It is a chromatographic technique used to separate the components in a mixture, to identify each component, and to quantify each component. In general, the method involves a liquid sample being passed over a solid adsorbent material packed into a column using a flow of liquid solvent. for the separation, identification, and quantification of the sample mixture.
High-performance liquid chromatography is now one of the analytical chemistry’s most powerful tools. Since it’s separate, identify and quantitate the analytes present in a sample mixture that may dissolve in a liquid.
All chromatographic separation, such as thin-layer chromatography (TLC), column chromatography, HPTLC, and paper chromatography works under the same basic principle. HPLC is mainly a highly advanced form of column chromatography.
The HPLC principle is based on the distribution of the component between a stationary phase (HPLC column) and a mobile phase (solvent). Depending on the chemical structure of the molecules they are retarded as passing the stationary phase.
The intermolecular interactions among a sample’s molecules and the packaging material determine their on-column period. Therefore, different components of a sample mixture are eluted at dissimilar retention times.
Each analyte in the sample interacts slightly differently with the adsorbent material, thus retarding the flow of the analytes. If the interaction is weak, the analytes flow off the column in a short amount of time, and if the interaction is strong, then the elution time is long.
¿How does work?
In very small amounts, the sample mixture to be separated and tested is sent into a stream of mobile phase percolating via a column. There are different types of columns available with sorbents of varying particle sizes and surfaces. The mixture moves through the column at varying velocities and interacts with the sorbent, also known as the stationary phase.


The velocity of each component in the mixture depends on:
1) Its chemical nature.
2) The nature of the column.
3) The composition of the mobile phase.
The time at which a specific analyte emerges from the column is termed as its retention time. The retention time is measured under specific conditions and considered as the identifying characteristic of a given analyte.
Sorbent particles might be hydrophobic or polar in nature. The commonly used mobile phases include any miscible combination of water and organic solvents such as acetonitrile and methanol. Water-free mobile phases can also be used.
Different types of HPLC


The following are the types of HPLC based on the stationary phase in the process:
Normal Phase HPLC: NP-HPLC separates the molecules according to polarity, in which the polar stationary phase and the non-polar mobile phase is used.
Reverse Phase HPLC: The reverse phase chromatography works on the principle of hydrophobic interactions so the more nonpolar the analyte has, the longer it will be retained. It this mobile phase is polar and the stationary phase is nonpolar in nature.
Size-Exclusion HPLC: Size Exclusion Chromatography (SEC) is a chromatographic process that separates molecules based solely on their size, in this technique molecules are separated by the column packing material on the basis of their exclusion from pores.
Ion-Exchange HPLC: It uses to separate the ions and polar molecules based on their affinity to the ion exchanger. Ion exchange chromatography is the most popular method for the purification of proteins and other charged molecules.
Experimental procedure of HPLC:
Before beginning an experiment, we must recognize the various components essential to perform the process.
HPLC Pump: The HPLC pump produces high pressure that gives a continuous and reproducible flow to the mobile phase throughout the HPLC system. E.g. Reciprocating pump, syringe pump, and pneumatic pump.
HPLC Mobile phase: It is a solvent or contains a combination of water with organic solvents, an ideal amount of an aqueous solution with polar solvents, or mixtures of organic solvent.
HPLC Degasser: It is a tool for removing gas from a mobile phase used in.
HPLC Injector: A sample injector is a device used to inject samples solution into the system. E.g. Rheodyne injector, septum injector, and stop flow injector.
HPLC Column: The column is the key component is responsible for separating the analytes of the sample mixture. Columns are now designed for use at high pressure in stainless steel tubes. Typically, silica gel is filled into the column known as the stationary phase.
HPLC Oven: It is a device used to control the temperature of a column.
HPLC Detector: A detector is a device used to detect compounds separated from a column. The detector transforms the effluent into an electrical signal and recorded by the computerized system. E.g. UV/VIS detector, PDA detector, Mass detector (LCMS), Fluorescence detector, and Infrared detector, etc.
Experimental Procedure


- Install column (C8 / C18) properly as required.
- Prepare the mobile phase and fill it in the reservoir. (Solvent, Buffer, or combination of it)
- Prepare samples in different concentrations, as needed.
- Build a method, and fill parameters such as flow rate, the mobile phase composition, wavelength, oven temperature, and program time.
- Create a sequence for samples and save it.
- Purge the mobile phase reservoir by opening the purging valve.
- Gradually increase system flow up to the required flow rate, and wait until the column is saturated and the baseline is corrected.
- As you get a baseline, then inject the sample manually or by auto-sampler via injector (sample loop/syringe).
- After analyzing the sample, study the retention time, tailing factor, capacity factor, and theoretical plates of each peak.
- Repeat the process according to the number of samples.
- Wash the column properly by HPLC grade water, and methanol/ acetonitrile.
HPLC Applications


- For pharmaceutical applications, is used to monitor drug stability, tablet dissolution analysis of pharmaceutical dosages form, and for quality control, etc.
- For environmental applications, is used to bio-monitoring of pollutants, and detection of phenolic compounds in drinking water, etc.
- For forensics applications, is used to the quantification of drugs in biological samples, determination of cocaine, steroids, and other abused drugs in blood, urine, etc.
- For clinical applications, is used to analysis of urine, bilirubin, antibiotics, etc. in blood.
- For food and beverage applications, is used to the analysis of polycyclic compounds in vegetables, analysis of preservative, sugar, and for measurement of quality of water and soft drinks.
The Advantages of HPLC are as follows.


- The high-performance liquid chromatography provides a simple, automated, and highly accurate method of identifying certain chemical components in a sample.
- Provides a quantitative and qualitative analysis that is simple and accurate.
- It can be upgrading to mass spectroscopy.
- Compared to other chromatographic techniques such as column chromatography, TLC, and paper chromatography, HPLC is fast, effective and delivers high resolution.
- The gradient elution is readily adaptable in HPLC.
The Disadvantages of HPLC are as follows.


- This requires a large number of expensive solvents, power supplies, and regular maintenance.
- Need to be expertise, since it is more difficult for beginners.
- The reliability of the separation process depends on the cleanliness of the mobile phase, sample and proper system operation.
- The contaminated column can affect the peak shapes.
Commonly asked questions on HPLC
What is the basic principle of HPLC? The basic principle is to separate the molecules between the stationary phase and the mobile phase. Since molecules will have different partition coefficients, on that basis they will be separated.
What are the types of HPLC? Normal phase HPLC, Reverse phase HPLC, Size-exclusion HPLC, and Ion-exchange HPLC, etc. are the types of HPLC based on the phase system (stationary) in the process.
What is the major advantage? One of the major advantages of high-performance liquid chromatography is that it has the ability to test a wide variety of samples.
What is the main difference between HPLC and HPTLC? The main difference between is that HPLC enables quantitative molecules separation in a sample mixture, while HPTLC does not permit.
What kind of precautions to take during analysis?
- Make sure the column washed before and after analysis.
- Solvents must be filtered through a 0.5 μm nylon filter membrane and degassed.
- The sample must be particle-free, therefore filtered through a 0.2 μm nylon filter membrane.
- Buffers like phosphate buffers, acetate buffers, etc. are very harmful to the HPLC system and columns they need to be washed properly.
- Don’t overload the column.
- Use appropriate flow rates to maintain system pressure.
- Do not run HPLC systems at high backpressure.
- Always use grade solvents and water derived from reliable sources.
- Use guard columns to protect against contamination and prolong column life.
- Don’t use a mobile phase or buffers with a highly acidic or basic pH.
Glossary of HPLC
The listing should be helpful to those just starting in HPLC but it also can serve as a refresher for long-time users in the field.
To see all list go here!
References
A. Wikipedia: https://en.wikipedia.org/wiki/High-performance_liquid_chromatography
B. Knauer: https://www.knauer.net/en/search?q=chromatography
C. Kromasil: https://www.kromasil.com/support/faq.php
D. Shimadzu: https://www.shimadzu.com/an/service-support/technical-support/analysis-basics/basic/what_is_hplc.html
E. ChemistryView: https://www.chemistryviews.org/details/education/9464911/What_is_HPLC/