¿What is HPLC? All about this chromatography advance!
¿What is HPLC?
HPLC stands for High-Performance Liquid Chromatography, formerly referred to as High-Pressure Liquid Chromatography.
It is a chromatographic technique used to separate the components in a mixture, to identify each component, and to quantify each component. In general, the method involves a liquid sample being passed over a solid adsorbent material packed into a column using a flow of liquid solvent. for the separation, identification, and quantification of the sample mixture.
High-performance liquid chromatography is now one of the analytical chemistry’s most powerful tools. Since it’s separate, identify and quantitate the analytes present in a sample mixture that may dissolve in a liquid.
All chromatographic separation, such as thin-layer chromatography (TLC), column chromatography, HPTLC, and paper chromatography works under the same basic principle. HPLC is mainly a highly advanced form of column chromatography.
The HPLC principle is based on the distribution of the component between a stationary phase (HPLC column) and a mobile phase (solvent). Depending on the chemical structure of the molecules they are retarded as passing the stationary phase.
The intermolecular interactions among a sample’s molecules and the packaging material determine their on-column period. Therefore, different components of a sample mixture are eluted at dissimilar retention times.
Each analyte in the sample interacts slightly differently with the adsorbent material, thus retarding the flow of the analytes. If the interaction is weak, the analytes flow off the column in a short amount of time, and if the interaction is strong, then the elution time is long.
¿How does work?
In very small amounts, the sample mixture to be separated and tested is sent into a stream of mobile phase percolating via a column. There are different types of columns available with sorbents of varying particle sizes and surfaces. The mixture moves through the column at varying velocities and interacts with the sorbent, also known as the stationary phase.


The velocity of each component in the mixture depends on:
1) Its chemical nature.
2) The nature of the column.
3) The composition of the mobile phase.
The time at which a specific analyte emerges from the column is termed as its retention time. The retention time is measured under specific conditions and considered as the identifying characteristic of a given analyte.
Sorbent particles might be hydrophobic or polar in nature. The commonly used mobile phases include any miscible combination of water and organic solvents such as acetonitrile and methanol. Water-free mobile phases can also be used.
Different types of HPLC


The following are the types of HPLC based on the stationary phase in the process:
Normal Phase HPLC: NP-HPLC separates the molecules according to polarity, in which the polar stationary phase and the non-polar mobile phase is used.
Reverse Phase HPLC: The reverse phase chromatography works on the principle of hydrophobic interactions so the more nonpolar the analyte has, the longer it will be retained. It this mobile phase is polar and the stationary phase is nonpolar in nature.
Size-Exclusion HPLC: Size Exclusion Chromatography (SEC) is a chromatographic process that separates molecules based solely on their size, in this technique molecules are separated by the column packing material on the basis of their exclusion from pores.
Ion-Exchange HPLC: It uses to separate the ions and polar molecules based on their affinity to the ion exchanger. Ion exchange chromatography is the most popular method for the purification of proteins and other charged molecules.
Experimental procedure of HPLC:
Before beginning an experiment, we must recognize the various components essential to perform the process.
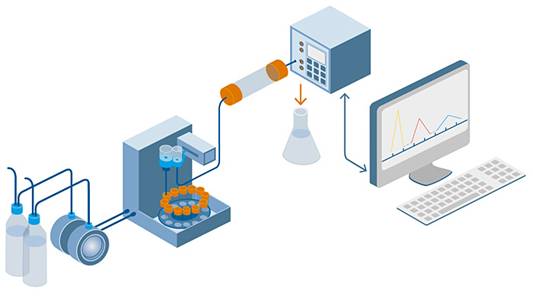
HPLC Pump: The HPLC pump produces high pressure that gives a continuous and reproducible flow to the mobile phase throughout the HPLC system. E.g. Reciprocating pump, syringe pump, and pneumatic pump.
HPLC Mobile phase: It is a solvent or contains a combination of water with organic solvents, an ideal amount of an aqueous solution with polar solvents, or mixtures of organic solvent.
HPLC Degasser: It is a tool for removing gas from a mobile phase used in.
HPLC Injector: A sample injector is a device used to inject samples solution into the system. E.g. Rheodyne injector, septum injector, and stop flow injector.
HPLC Column: The column is the key component is responsible for separating the analytes of the sample mixture. Columns are now designed for use at high pressure in stainless steel tubes. Typically, silica gel is filled into the column known as the stationary phase.
HPLC Oven: It is a device used to control the temperature of a column.
HPLC Detector: A detector is a device used to detect compounds separated from a column. The detector transforms the effluent into an electrical signal and recorded by the computerized system. E.g. UV/VIS detector, PDA detector, Mass detector (LCMS), Fluorescence detector, and Infrared detector, etc.
Experimental Procedure


- Install column (C8 / C18) properly as required.
- Prepare the mobile phase and fill it in the reservoir. (Solvent, Buffer, or combination of it)
- Prepare samples in different concentrations, as needed.
- Build a method, and fill parameters such as flow rate, the mobile phase composition, wavelength, oven temperature, and program time.
- Create a sequence for samples and save it.
- Purge the mobile phase reservoir by opening the purging valve.
- Gradually increase system flow up to the required flow rate, and wait until the column is saturated and the baseline is corrected.
- As you get a baseline, then inject the sample manually or by auto-sampler via injector (sample loop/syringe).
- After analyzing the sample, study the retention time, tailing factor, capacity factor, and theoretical plates of each peak.
- Repeat the process according to the number of samples.
- Wash the column properly by HPLC grade water, and methanol/ acetonitrile.
HPLC Applications


- For pharmaceutical applications, is used to monitor drug stability, tablet dissolution analysis of pharmaceutical dosages form, and for quality control, etc.
- For environmental applications, is used to bio-monitoring of pollutants, and detection of phenolic compounds in drinking water, etc.
- For forensics applications, is used to the quantification of drugs in biological samples, determination of cocaine, steroids, and other abused drugs in blood, urine, etc.
- For clinical applications, is used to analysis of urine, bilirubin, antibiotics, etc. in blood.
- For food and beverage applications, is used to the analysis of polycyclic compounds in vegetables, analysis of preservative, sugar, and for measurement of quality of water and soft drinks.
The Advantages of HPLC are as follows.


- The high-performance liquid chromatography provides a simple, automated, and highly accurate method of identifying certain chemical components in a sample.
- Provides a quantitative and qualitative analysis that is simple and accurate.
- It can be upgrading to mass spectroscopy.
- Compared to other chromatographic techniques such as column chromatography, TLC, and paper chromatography, HPLC is fast, effective and delivers high resolution.
- The gradient elution is readily adaptable in HPLC.
The Disadvantages of HPLC are as follows.


- This requires a large number of expensive solvents, power supplies, and regular maintenance.
- Need to be expertise, since it is more difficult for beginners.
- The reliability of the separation process depends on the cleanliness of the mobile phase, sample and proper system operation.
- The contaminated column can affect the peak shapes.
Commonly asked questions on HPLC
What is the basic principle of HPLC? The basic principle is to separate the molecules between the stationary phase and the mobile phase. Since molecules will have different partition coefficients, on that basis they will be separated.
What are the types of HPLC? Normal phase HPLC, Reverse phase HPLC, Size-exclusion HPLC, and Ion-exchange HPLC, etc. are the types of HPLC based on the phase system (stationary) in the process.
What is the major advantage? One of the major advantages of high-performance liquid chromatography is that it has the ability to test a wide variety of samples.
What is the main difference between HPLC and HPTLC? The main difference between is that HPLC enables quantitative molecules separation in a sample mixture, while HPTLC does not permit.
What kind of precautions to take during analysis?
- Make sure the column washed before and after analysis.
- Solvents must be filtered through a 0.5 μm nylon filter membrane and degassed.
- The sample must be particle-free, therefore filtered through a 0.2 μm nylon filter membrane.
- Buffers like phosphate buffers, acetate buffers, etc. are very harmful to the HPLC system and columns they need to be washed properly.
- Don’t overload the column.
- Use appropriate flow rates to maintain system pressure.
- Do not run HPLC systems at high backpressure.
- Always use grade solvents and water derived from reliable sources.
- Use guard columns to protect against contamination and prolong column life.
- Don’t use a mobile phase or buffers with a highly acidic or basic pH.
Glossary of HPLC
The listing should be helpful to those just starting in HPLC but it also can serve as a refresher for long-time users in the field.
To see all list go here!
References
A. Wikipedia: https://en.wikipedia.org/wiki/High-performance_liquid_chromatography
B. Knauer: https://www.knauer.net/en/search?q=chromatography
C. Kromasil: https://www.kromasil.com/support/faq.php
D. Shimadzu: https://www.shimadzu.com/an/service-support/technical-support/analysis-basics/basic/what_is_hplc.html
E. ChemistryView: https://www.chemistryviews.org/details/education/9464911/What_is_HPLC/